Global Journal of Fertility and Research
Pollen Characteristics of Diploid and Tetraploid Grape Cultivars
Zeki Kara1*, Mohammed Salah Mohammednoori Fakhar2 and Kevser Yazar1
2Department of Horticulture, Selçuk University, Graduate School of Natural and Applied Sciences, 42075, Konya, Turkey
Cite this as
Kara Z, Mohammednoori Fakhar MS, Yazar K. Pollen Characteristics of Diploid and Tetraploid Grape Cultivars. Glob J Fertil Res. 2024; 9(1): 001-007. Available from: 10.17352/gjfr.000025Copyright
© 2024 Kara Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.For a successful grapevine breeding program, pollen viability, germination ability, and cultivar compatibility information are of vital importance. In this study, pollen viability, germination rates, pollen tube lengths, and pollen tube diameters of three grape cultivars [Kyoho (4n), Ekşi Kara (2n), and Gök Üzüm (2n)] were investigated. Pollen viability rates were determined using the IKI (iodine potassium iodide) staining test. In vitro, pollen germination rates were determined using a semi-solid medium containing 20% sucrose and 1% agar. As a result, significant differences were detected in pollen viability, germination rates, and pollen tube lengths of the examined grape cultivars. The first germination started at the 48th hour in all three grape cultivars. In cv. Ekşi Kara had the highest pollen viability while the lowest (3.05%) value in terms of pollen germination was determined. Pollen tube length and pollen tube diameter of the cv. Kyoho had higher values than the other two diploid cultivars. Pollen tube formation started in the first 48th hours, while pollen tube growth continued to increase until the 96th hours. As a result, it was confirmed that the flower structure of the cv. Ekşi Kara was functionally female. In suitable ecologies, diploid cv. Gök Üzüm and tetraploid cv. Kyoho were found to be usable as pollinators.
Introduction
The grapevine is one of the most widely grown and economically important species globally. It is grown in a wide area from 50° latitude to the tropics in the northern hemisphere and to 43° latitude in the southern hemisphere [1]. 85.132529 million tons of grapes are produced annually in 67.30179 million hectares of vineyard area in the world [2].
Approximately 4 million tons of grapes are produced annually from 0.43 million hectares of vineyard area surface in Turkey. 1.99 million tons of grapes produced are table grapes, 1.54 million tons are dried grapes, and 0.47 million tons are wine grapes [3]. A total of 1435 grape varieties collected from across the country and bred in recent years have been protected in the Turkish grapevine gene bank [4]. Despite this density in grape diversity, local and global markets and grape producers demand new varieties with superior characteristics.
Grape varieties developed by hybridizing Vitis vinifera L. varieties with each other and with other species constitute almost all the global grape production. Although some of the grape varieties have become international and are produced in many countries, grape variety development studies are actively carried out in most of the important wine-growing countries.
For example, the cv. Kyoho, which was developed in Japan in 1937 and released in 1946, has reached the largest vineyard area on a global scale. Cv. Cabernet Sauvignon, on the other hand, is a white wine variety that has become international and has the largest vineyard area globally [5]. Red Globe, bred in 1958, is the best-selling, red colour, seeded table grape variety in the world, and Italia, bred in 1911, is the best-selling white-seeded table grape variety in the international market [2,6].
Priorities in the development of grape varieties are increasing biotic and abiotic stress tolerance, reducing pesticide requirements, and improving yield and quality. In particular, the table grape market brings dynamism to the need for new grape varieties. In this context, table grape-producing countries need to develop new varieties to maintain and increase their market shares. In Turkey, efforts are being made to develop new superior varieties through hybridization and other breeding methods to protect and increase the share of the viticulture sector in the international market [7-9].
Pollen of grapevine and grape varieties is the subject of many studies because it affects fruit set. Characterization of cultivars by pollen grains has been frequently studied. Pollen morphology and germination are important indicators for taxonomic purpose and productivity. Knowing the pollen characteristics of grape varieties is a necessity for grape producers and especially vine breeders [10-12].
Grape production is related to pollen fertility, which depends on pollen viability and germination potential [13]. Pollen contains the male gametophyte and is produced in the anthers of higher plants, and its main role is the sexual reproduction of spermatophytes [14].
Pollen quality, also often called pollen fertility, is determined by a combination of different characteristics such as the viability of mature pollen and the germination ability of pollen under in vitro conditions, pollen tube formation, and growth [15]. These can be influenced by genetics, environmental (temperature and humidity) and agronomic factors [1].
Evaluation of pollen quality is important in various aspects such as examining the storage potential of pollen grains for controlled pollination, evaluation of intra- and inter-varietal incompatibility, clonal selection and genetic breeding experiments [16], and identification of some agronomic practices to increase pollen productivity [17-19].
Pollen quality plays a critical role in grape production and affects the fruit setting rate. Grape pollen efficiency depends on its viability and germination potential [13]. Palynological studies are needed before designing hybridization programs for the development of rootstock and scion varieties [20,21].
Studies have shown that toxic substances such as pesticides, plant bio-stimulants, and chemical fertilizers used extensively in agriculture damage plant pollen viability and cause a decrease in productivity. Pollen amount, viability, and germination ability are important parameters for both productivity and breeding studies [19].
While pollen viability is determined by staining in 2,3,5 Triphenyl Tetrazolium Chloride (TTC), Fluorescein Diacetate (FDA), and Trypan Blue (TB) solutions, pollen germination experiments in vitro can be performed using agar in a petri dish [22].
Kelen and Demirtas [23], the viability, germination ability, and production levels of pollen of various grape varieties (Vitis vinifera L.) grown in Isparta were examined under in vitro conditions. The vitality levels of pollen ranged between 23.8% and 80.8% in the FDA (fluorescein diacetate) test and between 31.5% and 68.8% in the TTC (2,3,5-triphenyl tetrazolium chloride) test. According to pollen germination rates, the most suitable medium in the hanging drop method and saturated petri dish method was 20% sucrose concentration and 1% agar + 15% sucrose solution, respectively.
Sabir [19], examined the effects of nano-sized calcite and seaweed extract applications on the pollen size, productivity, and germination rates of cvs. Thompson Seedless and Narince. Calcite application, when done alone or in combination with seaweed, increased viable pollen percentages from 65% to 77.9% in the cv. Thompson Seedless and from 55.3% to 68.8% in the cv. Narince. The improvement in germination rate due to calcite in the pollen of the cv. Narince was found to be more evident than that of the cv. Thompson Seedless. In response to the treatments, slight changes in the lengths of the polar and equatorial axes of grape pollen were observed. The correlation between pollen viability and germination percentages was found to be different among varieties.
Kara, et al. [24], in a study in which they examined the self-fertility among 220 clone candidates selected in the clone selection study of the Ekşi Kara grape variety, self-pollinated by closing the flower clusters, and free-pollinated with the Gök Üzüm variety, and examined the viability, germination, and tube growth of the pollen, they found that not all clone candidates formed seeded berries, and the pollen most of them were viable (90.67%) but they found the germination rate to be less than 3%.
Pereira, et al. [1], pollen quality of 15 Vitis vinifera L. varieties was examined. Pollen viability was determined by fluor chromatic reaction and germination rates were examined in vitro in two different environments. In the viability test, viability was over 50% in 13 varieties, and over 75% in 8 varieties, pollen germination rates showed significant differences among grape varieties, and low (below 14%) in three varieties (cvs. Touriga Nacional, Cabernet Franc, and Cabernet Sauvignon), high germination rates were determined in three varieties (cvs. Castelão, Loureiro, Malbec and Petit Verdot), but no significant difference could be determined between the germination percentages of the examined varieties in two different environments.
Burçak [25], examined the pollen morphology of ten grape varieties with a scanning electron microscope and determined significant differences between varieties in the width (10.12 - 22.44 µm), length (16.26 - 29.91 µm), and L/W ratio (1.08 - 2.55) of pollens. In cvs. Alicante Boushet, Cardinal, and Syrah, groove-less pollens surrounded within the exine were observed, while in other varieties, areolate pollens were observed. TTC tests identified significant differences in pollen viability between varieties, ranging from 11.75% - 84.25%.
In grapevines and other cultivated plant species, the presence of sufficient pollinators increases fruit yield and product quality [26-28]. Especially in grape varieties with functional female flower structures, fruit formation requires pollination of the ovary with viable and germinable pollen to develop into a functional fruit. Pollination and subsequent fertilization prevent flower shedding thanks to the synthesis of various phytohormones [29] and accelerate fruit setting and fruit development [30].
Although studies on in vitro pollen germination and pollen tube development have been conducted with several cultivars [23,31-33], in this study, the in vitro characterization of pollinator qualities of cv. Gök Üzüm and cv. Kyoho was studied, especially for the cv. Ekşi Kara, which has a functional female flower.
Material and method
Plant material
In the trial conducted in 2023 at the Horticulture Department of Selçuk University Faculty of Agriculture, pollen of cvs. Ekşi Kara, Gök Üzüm (2n, V. vinifera L.) which are best adapted to the areas suitable for viticulture in the Central Taurus Mountains, in the clone comparison vineyard and cv. Kyoho (4n, Vitis interspecific hybrid) in the greenhouse was used.
Ekşi Kara (Vitis vinifera L.) is an old and autochthonous grape variety grown intensively in Konya, well adapted to the ecology and high yield. Therefore, it is promising in vineyard areas with similar ecology. Its flowers are functionally female and need a pollinator variety for a good fruit set. Cv. Gök Üzüm (Vitis vinifera L.) is another autochthonous variety of the region and is used as a main pollinator for cv. Ekşi Kara in the region due to its hermaphrodite flowers [24,34].
The cv. Kyoho was bred in Japan by crossing cv. Ishihara wase and cv. Centennial. It is a popular variety in Japan, Taiwan, China, and Korea due to its large (12 g - 14 g), foxy-flavoured, very sweet (18 - 20 °Brix) berries, and its popularity is increasing all over the world. It is resistant to diseases and seedless berries can be produced with GA applications. It is also used as a gene source in breeding studies in Turkey [5,35].
Pollen collection
A week before flowering, the inflorescences were covered with paper bags (Figure 1). Bagged inflorescence of three vines of each genotype was monitored regularly, pollen was collected between 7 am - 9 am during the full bloom period (EL 23, when 50% of the flower heads have fallen) [36]. Fifteen inflorescences of each grape cultivars used in the experiment were used as pollen sources. It was dried in the laboratory at room temperature for 24 hours and sieved to remove flower residues [37].
Pollen viability
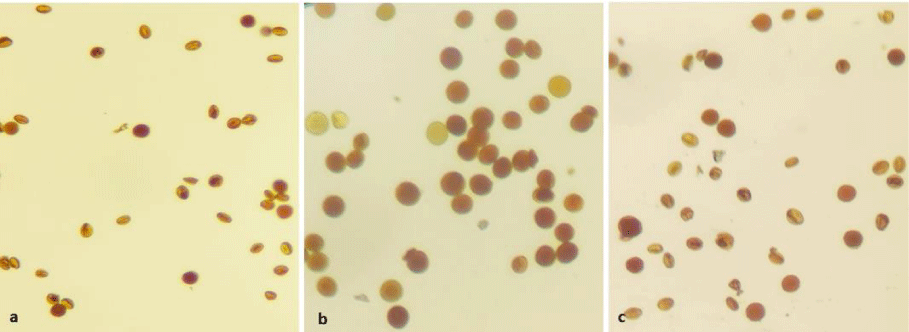
IKI (iodine potassium iodide) staining test was used to determine pollen viability. A drop of IKI solution was dropped on a slide, and the pollen grains were spread on the slide with a brush and covered with a coverslip. In determining pollen viability, pollens that turned orange or bright red under a light microscope were considered alive. Three randomly selected microscopic fields (each containing at least 100 pollen grains) were used to determine the viability of pollen on each slide.
In vitro pollen germination
An in vitro pollen germination test was performed on a standardized solid medium containing 20% sucrose + 1% agar at pH 5.85. A small rectangular piece of media was removed with a scalpel and placed on a slide. Pollen grains were spread evenly on the surface of the medium with a fine brush. The slides were placed in a petri dish with moist filter paper at the bottom and covered with a lid to prevent the medium from drying out and maintain humidity. Petri dishes were kept in a dark environment for incubation. After 48th, 72nd, and 96th hours of incubation, pollen tube development in the environment was recorded under a light microscope. Pollen was considered germinated when the pollen tube length exceeded the pollen size [38]. The pollen germination rate was calculated by counting three fields, each containing at least 100 pollen grains.
Statistical analysis
The trial design was random parcels, and the applications performed at each stage were evaluated in discrete trials by analysis of variance analysis and Tukey comparison tests in the JMP7 statistical program [39].
Results and discussion
Pollen viability
Pollen viability varied according to varieties. The highest pollen viability was determined in cv. Ekşi Kara (84.12%), the lowest pollen viability was determined in cv. Gök Üzüm (77.61%), and viable pollen rates were high in all three cultivars (Table 1, Figure 2).
In a similar study, Kelen and Demirtas [23] performed an in vitro viability test of Vitis vinifera L. pollen and found the viability rates of the pollen to be between 23.8% and 80.8% in the FDA (fluorescein diacetate) test, and TTC (2, They determined it in the range of 31.5% - 68.8% in the 3,5-triphenyl tetrazolium chloride) test.
Korkutal, et al. [40], when they examined the pollen viability rate of 23 grape varieties during the full bloom period, found the highest in the cv. Trakya İlkeren and cv. Italian (100%), and the lowest in the cv. Chardonnay (54.8%).
Sabir [19], increased the viability and germination rates of pollen of cv. Thompson Seedless and cv. Narince grape varieties with nano calcite and seaweed extract applications. It was determined that when seaweed and calcite were applied together, the live pollen rate increased from 65% to 77.9% in the cv. Thompson Seedless and from 55.3% to 68.8% in the cv. Narince variety, compared to the application of calcite alone.
Kara, et al. [24], determined pollen viability rates between 38.5% and 96.83% using the IKI (iodinated potassium iodide) staining test in cv. Ekşi Kara clones.
Pollen germination
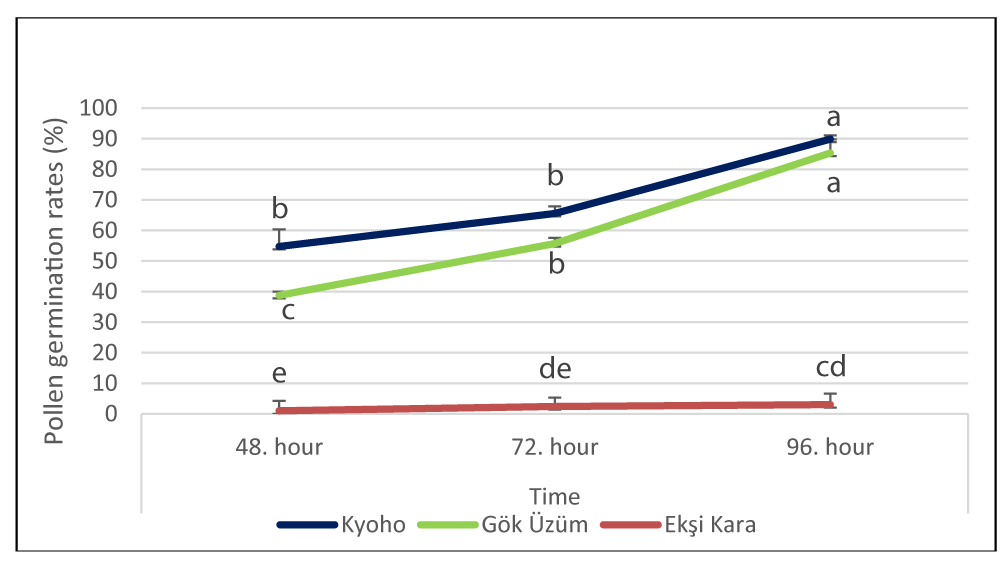
The change in pollen germination rates (%) according to varieties during the pollen germination process, at the 48th, 72nd, and 96th hours, the highest germination rate was determined in the cv. Kyoho (54.77%, 65.54%, and 89.86%), while it was 38%, 55.70%, and 85.32% in the cv. Gök Üzüm, and in the cv. Ekşi Kara was determined as 1.03%, 2.4%, and 3.05%, respectively (Figure 3).
While the average pollen germination rate was highest in the cv. Kyoho (70.06%), the lowest value was recorded in the cv. Ekşi Kara (2.28%). Pollen germination rates increased over time in all varieties. While the highest value of pollen viability was recorded in the cv. Ekşi Kara variety (84.12%), the pollen germination rate was quite low (1.03% - 3.05%).
Previous studies have reported differences in pollen viability, pollen germination, and pollen tube growth in different species according to varieties [23,41,42]. A direct relationship between pollen viability and germination ability has been observed in many species [43-45].
Pereira, et al. [1], pollen quality of 15 Vitis vinifera L. varieties was examined. Pollen germination rates varied greatly depending on the grape cultivars examined. Cvs. While Touriga Nacional, Cabernet Franc, and Cabernet Sauvignon gave low germination values (below 14%) in two different germination environments, Cvs. Castelão Loureiro, Malbec and Petit Verdot had high germination rates (over 40%).
Kara, et al. [24], found the germination rate of the cv. Ekşi Kara pollen to be less than 3% in the medium containing 20% sucrose + 1% agar.
Baby, et al. [46] recorded in vitro pollen germination rates of cvs. Shiraz, Cabernet Sauvignon, and Merlot as approximately 75%, 50%, and 25%, respectively.
Pollen tube length
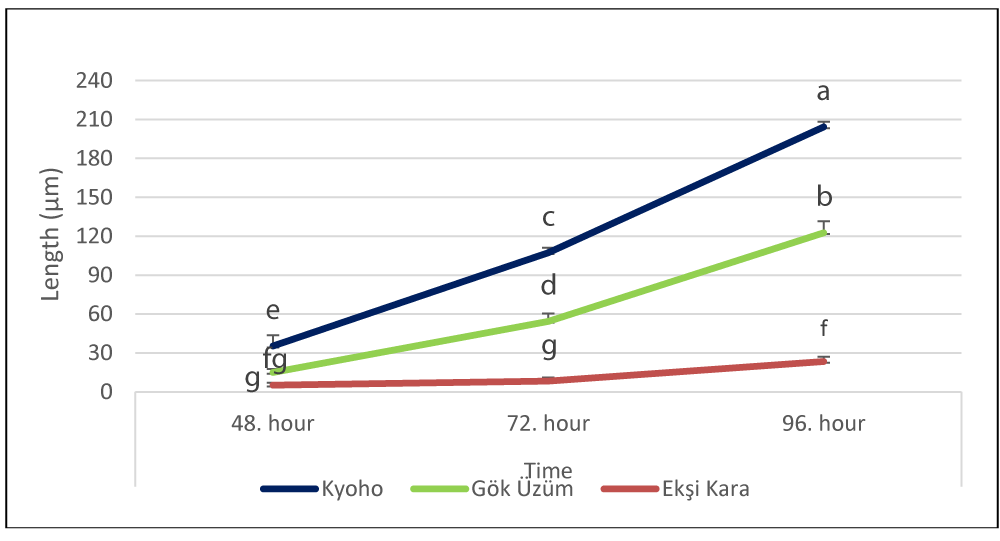
During the pollen germination process, pollen tube lengths according to varieties were measured as 35.33 µm, 107.33 µm, and 204.33 µm at the 48th, 72nd, and 96th hours in cv. Kyoho, 15.00 µm, 54.33 µm and 122.67 µm in cv. Gök Üzüm variety and 5.13 µm, 8.33 µm and 23.40 µm in cv. Ekşi Kara, respectively. An increase in pollen tube length was recorded as the germination period extended in all three varieties (Figure 4). The ranking in terms of average pollen tube lengths was as follows: Kyoho (115.66 µm), Gök Üzüm 64.00 µm and Ekşi Kara (12.28 µm).
In a previous study, Baby, et al. [46] determined that the pollen tube length of cv. Shiraz was higher than that of cvs. Cabernet Sauvignon and Merlot. They determined that approximately 45% of the pollen tubes in cv. Shiraz extended more than 500 μm, but only 12% and 4% of the pollen tubes in cvs. Cabernet Sauvignon and Merlot reached a length of more than 500 μm. They considered that the longer growth of pollen tubes in cv. Shiraz played a functional role because the distance from the stigma surface to the ovary was longer than in other cultivars.
In another study, Padureanu and Patras [47] reported that in vitro, the pollen germination rate in cv. Othello was higher than in cv. Noah, pollen tube growth was faster in cv. Othello, and pollen tube growth increased significantly in the first 24 hours in media containing 15% and 25% sucrose.
In another similar study, Korkutal, et al. [40] investigated the in vitro pollen germination rate in 23 grape varieties and determined low germination rates in cv. Chardonnay (19.675%), cv. Pinot Noir (23.450%) and cv. Gamay (30.025%) varieties, and high germination rates in cvs. Boğazkere (81.400%) and Clairette (80.925%).
Germinating pollen grains of the cv. Ekşi Kara in a medium containing 20% sucrose + 1% agar, Kara, et al. [24] determined the longest pollen tube length as 123.22 µm in germinated pollen grains.
Pollen tube diameter
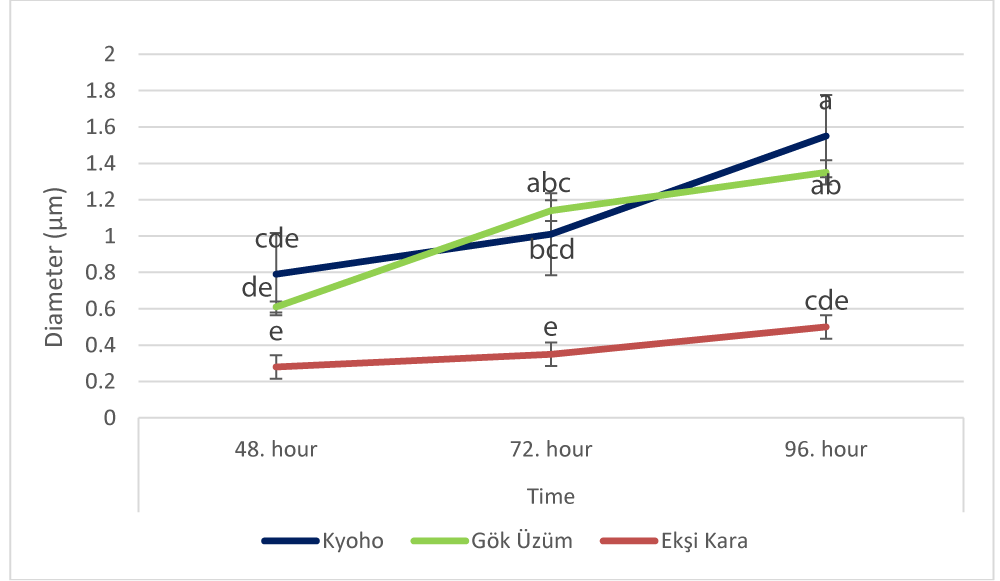
Significant increases in pollen tube diameter were recorded according to the varieties during the pollen germination process (Figure 5), the change was recorded as 0.79 µm, 1.01 µm, and 1.55 µm in cv. Kyoho, 0.61 µm, 1.14 µm and 1.35 µm in cv. Gök Üzüm and 0.28 µm, 0.35 µm and 0.50 µm in cv. Ekşi Kara at the 48th, 72nd, and 96th hours, respectively.
Pollen tube diameter is an important factor affecting the rate of pollen tube growth and fertilization success. The diameters of pollen tubes can vary depending on genetic factors, environmental conditions, and plant species. Different diameters can usually be observed in different grape varieties. Pollen tube diameters can affect the growth rate and fertilization success of pollen tubes. Previous studies examining plant physiology and fertilization processes have indicated that pollen tube diameters generally vary between 3 µm - 12 µm [37,48,49]. Sharafi [44] examined the pollen tube growth rates and diameters of 15 different grape varieties in a study. He observed significant differences among varieties and determined the pollen tube diameters to be between 5 µm - 15 µm. The results we obtained in this study were like the literature examining the pollen tube.
Conclusion
The limitation of this study was that it was conducted in vitro on pollen samples taken from two diploids (Ekşi Kara and Gök Üzüm, both are Vitis vinifera L.) and one tetraploid (Kyoho Vitis complex) grape cultivars grown at 1200 m above sea level. It should be noted that pollen viability and productivity are affected by the pollen source grape varieties, growing conditions, and the environment in which the pollen was germinated.
According to the findings obtained in this study, alive pollen rates were determined as 77.61% (cv. Gök Üzüm), 80.00% (cv. Kyoho), and 84.12% (cv. Ekşi Kara) according to the cultivars. There was no direct relationship between pollen viability and germination rates. While the highest alive pollen value was determined in the cv. Ekşi Kara, the germinated pollen rate in this variety was the lowest and reached only 3.05% at the 96th hour.
The tetraploid cv. Kyoho had larger pollen tube length and pollen tube diameter than the diploid cvs. Ekşi Kara and Gök Üzüm. This may be attributed to the fact that cv. Kyoho, unlike the other two Vitis vinifera L. cultivars, has both Vitis labrusca L. and Vitis vinifera L. genes.
The pollens of cvs. Kyoho and Gök Üzüm were successfully germinated in 20% sucrose + 1% agar medium. Although a period of 48 hours was sufficient to determine the pollen germination rates, germination periods up to 96 hours were used to examine the pollen tube length and thickness to make the differences between the varieties clear.
The germination rates of the cv. Ekşi Kara was insufficient for a normal berry set. It was once again confirmed that the flower structure of this cultivar is functionally female. While cv. Gök Üzüm was used as a pollen source for cv. Ekşi Kara in traditional vineyard areas, cv. Kyoho was also found to be an efficient pollen source when the flowering process overlaps in open field conditions.
The flower structure of Ekşi Kara grape variety was hermaphrodite, and the highest live pollen rate was determined in this variety in pollen bush tests, but because of the pollen germination test, it was confirmed that the flower structure of this variety was functionally female. The necessity of pollen germination tests to determine the flower structures of grape varieties was confirmed.
By knowing the pollen characteristics in grape cultivars, fertilization, fruit setting, and fruit quality can be increased. At the same time, basic data can be provided for the development of local varieties through hybridization.
- Pereira MR, Ribeiro H, Cunha M, Abreu I. Comparison of pollen quality in Vitis vinifera L. cultivars. Scientia Horticulturae. 2018;227:112-116. Available from: https://doi.org/10.1016/j.scienta.2017.09.038
- FAO. World Food and Agriculture – Statistical Yearbook 2024. Rome, Italy: FAO; 2024.
- Turkish Statistical Institute (TÜİK). Available from: http://www.tuik.gov.tr/PreTablo.do?alt_id=1001. Accessed August 2017.
- Ergönül O, Özer C. Yeni geliştirilen üzüm çeşitleri and kullanımları. TZOB, Çiftçi ve Köy Dünyası. 2017;303:51-55. Available from: https://www.researchgate.net/publication/322520142_YENI_GELISTIRILEN_UZUM_CESITLERI_VE_KULLANIMLARI
- OIV. Distribution of the world’s grapevine varieties. Paris, France: OIV; 2017. Available from: https://www.oiv.int/public/medias/5888/en-distribution-of-the-worlds-grapevine-varieties.pdf
- VIVC. Vitis International Variety Catalogue. Available from: http://www.vivc.de/index.php.
- Barış C. New, seedless and early table grape varieties registered and put into production. Ministry of Agriculture and Rural Affairs, Agriculture and Village Magazine. 1992;82:52-53.
- Uslu İ, Samancı H, Demiray T, Gökçay E. Obtaining new table grape varieties through hybridization. Atatürk Horticulture Central Research Institute, Scientific Research and Investigations. 1995;56.
- Tuylu M. Vitis vinifera L. cv. Examination of meiosis defects in Uslu anthers. [Master's thesis]. Institute of Science and Technology; 2007; 55.
- Lombardo G, Carraro L, Cargnello G, Bassi M. Ultrastructure of pollen of Vitis vinifera L. cv “Picolit giallo” and its behaviour in experiments of self- and cross-pollination. Vitis. 1976;15:73-1581. Available from: https://core.ac.uk/download/pdf/235693716.pdf
- Carmona MJ, Chaïb J, Martínez-Zapater JM, Thomas MR. A molecular genetic perspective of reproductive development in grapevine. J Exp Bot. 2008;59:2579-2596. Available from: https://doi.org/10.1093/jxb/ern160
- Bautista J, Dangl GS, Yang J, Reisch B, Stover E. Use of genetic markers to assess pedigrees of grape cultivars and breeding program selections. Am J Enol Vitic. 2008;59:248-254. Available from: http://dx.doi.org/10.5344/ajev.2008.59.3.248
- Lombardo G, Cargnello G, Bassi M, Gerola F, Carrano L. Pollen ultrastructure in different vine cultivars with low productivity. Vitis. 2016;17:221. Available from: https://doi.org/10.5073/vitis.1978.17.221-228
- Pérez MT, Caballero MM, Sánchez JG, Criado MR, Fernández SD, Artero BC. Pollen in the atmosphere of Vélez-Málaga. Department of Environment. Vélez-Málaga City Council; 2007.
- Linskens HF, Stanley RG. Pollen: biology, biochemistry, management. Berlin, Heidelberg: Springer-Verlag; 1974. Available from: https://link.springer.com/book/10.1007/978-3-642-65905-8
- Dafni A, Firmage D. Pollen viability and longevity: practical, ecological and evolutionary implications. In: Pollen and Pollination. 2000; 113-132. Available from: https://link.springer.com/article/10.1007/BF00984098
- Caliskan O, Bayazit S, Ilgin M, Karatas N. Morphological diversity of caprifig (Ficus carica var. caprificus) accessions in the eastern Mediterranean region of Turkey: Potential utility for caprification. Scientia Horticulturae. 2017;222:46-56. Available from: http://dx.doi.org/10.1016/j.scienta.2017.05.008
- Novara C, Ascari L, La Morgia V, Reale L, Genre A, Siniscalco C. Viability and germinability in long-term storage of Corylus avellana pollen. Scientia Horticulturae. 2017;214:295-303. Available from: https://doi.org/10.1016/j.scienta.2016.11.042
- Sabir A. Improvement of the pollen quality and germination levels in grapes (Vitis vinifera L.) by leaf pulverizations with nanosize calcite and seaweed extract (Ascophyllum nodosum). JAPS: Journal of Animal & Plant Sciences. 2015;25. Available from: https://www.thejaps.org.pk/docs/v-25-06/15.pdf
- Caporali E, Spada A, Marziani G, Failla O, Scienza A. The arrest of development of abortive reproductive organs in the unisexual flower of Vitis vinifera ssp. silvestris. Sex Plant Reprod. 2003;15:291-300. Available from: http://dx.doi.org/10.1007/s00497-003-0169-5
- Abreu I, Costa I, Oliveira M, Cunha M, De Castro R. Ultrastructure and germination of Vitis vinifera cv. Loureiro pollen. Protoplasma. 2006;228:131-135. Available from: https://doi.org/10.1007/s00709-006-0167-1
- Gozlekci S, Kaynak L. Investigations on pollen production and quality in some standard pomegranate (Punica granatum L.) cultivars. Options Méditerranéennes. Série A, Séminaires Méditerranéens. 2000;71-77. Available from: https://om.ciheam.org/article.php?IDPDF=600254
- Kelen M, Demirtas I. Pollen viability, germination capability and pollen production level of some grape varieties (Vitis vinifera L.). Acta Physiol Plant. 2003;25:229-33. Available from: https://link.springer.com/article/10.1007/s11738-003-0002-7
- Kara Z, Sabir A, Yazar K, Doğan O, Khaleel AJK. Fertilization biology of ancient grapevine variety ‘Ekşi Kara’ (Vitis vinifera L.). Selcuk J Agric Food Sci. 2017;31:92-97. Available from: http://dx.doi.org/10.15316/SJAFS.2017.25
- Burçak İ. Pollen characteristics of some grape cultivars (Vitis vinifera L.). Int J Agric Environ Food Sci. 2021;5:279-286. Available from: https://doi.org/10.31015/jaefs.2021.3.4
- Pashte V, Kulkarni S. Role of pollinators in qualitative fruit crop production: a review. Trends Biosci. 2015;8:3743-9. Available from: https://www.researchgate.net/publication/281773883_Role_of_Pollinators_in_Qualitative_Fruit_Crop_Production_A_Review
- Sgarbi E, Bignami C, Meglioraldi S, Storchi M, Silvestroni O, Barbieri C, et al. Pollination and fruit set: critical factors for the production of the grapevine cultivar 'Malbo Gentile'. In: XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on Plant 932. 2010; 155-162. Available from: https://doi.org/10.17660/ActaHortic.2012.932.23
- Jovanović-Cvetković T, Šutalo V, Kupe M, Ercisli S, Životić A, Pašalić B. Influence of interaction effects of the different pollenizers on the Blatina variety (Vitis vinifera L.) grape cluster and seed characteristics. Plants. 2022;11:420. Available from: https://doi.org/10.3390/plants11030420
- Kühn N, Arce-Johnson P. Pollination: a key event controlling the expression of genes related to phytohormone biosynthesis during grapevine berry formation. Plant Signal Behav. 2012;7:7-11. Available from: https://doi.org/10.4161/psb.7.1.18353
- Godoy F, Kühn N, Muñoz M, Marchandon G, Gouthu S, Deluc L, Delrot S, Lauvergeat V, Arce-Johnson P. The role of auxin during early berry development in grapevine as revealed by transcript profiling from pollination to fruit set. Hortic Res. 2021;8. Available from: https://www.nature.com/articles/s41438-021-00568-1
- Gokbayrak Z, Engin H. Effect of plant growth regulators on in vitro pollen germination of grapevine cultivars. In: III Balkan Symposium on Fruit Growing 1139. 2015; 405-408. Available from: http://dx.doi.org/10.17660/ActaHortic.2016.1139.70
- Carreño J, Oncina R, Carreño I. In vitro studies on pollen germination capability and preservation of different cultivars of Vitis vinifera L. In: IX International Conference on Grape Genetics and Breeding 827. 2006; 493-496. Available from: https://doi.org/10.17660/ActaHortic.2009.827.85
- Gozlekci S, Kaynak L. Investigations on pollen production and quality in some standard pomegranate (Punica granatum L.) cultivars. Options Méditerranéennes. 2000;42:71-78. Available from: https://www.semanticscholar.org/paper/Investigations-on-pollen-production-and-quality-in-Gozlekci-Kaynak/d6f45f7188fd01309a9e3f7dff71d8bb2bfc8039
- Kara Z, Öz MH, Sabır A, Yazar K, Doğan O, Khaleel AJK, et al. Clonal preselection in grape (Vitis vinifera L.) varieties of Ekşi Kara and Gök Üzüm. Selcuk J Agric Food Sci. 2023;37:444-456. Available from: https://dergipark.org.tr/tr/download/article-file/3627933
- Kara Z. Ampelography, Selcuk University, Konya: Ders kitabı; 2022; 290.
- Lorenz DH, Eichhorn KW, Bleiholder H, Klose R, Meier U, Weber E. Growth stages of the grapevine: Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)—Codes and descriptions according to the extended BBCH scale. Aust J Grape Wine Res. 1995;1(2):100-103. Available from: http://dx.doi.org/10.1111/j.1755-0238.1995.tb00085.x
- Rane P, Thakre M, Verma MK, Prakash J, Kumar C, Srivastava V, et al. Standardization of pollen collection method and in vitro pollen germination media in grapes. Pharma Innov J. 2023;12:3159-3161. Available from: https://www.thepharmajournal.com/archives/2023/vol12issue12/PartAM/12-12-328-959.pdf
- Nepi M, Franchi G. Cytochemistry of mature angiosperm pollen. In: Pollen and Pollination. 2000; 45-62. Available from: https://link.springer.com/article/10.1007/BF00984095
- Yue Y, Zhu Y, Fan X, Hou X, Zhao C, Zhang S, et al. Generation of octoploid switchgrass in three cultivars by colchicine treatment. Ind Crops Prod. 2017;107:20-21. Available from: http://dx.doi.org/10.1016/j.indcrop.2017.05.021
- Korkutal İ, Bahar E, Demir K, Çelik S, Selen U. Examination of pollen viability and germination abilities of some grape varieties (Vitis vinifera L.) using in vitro tests. Trakya Univ J Sci. 2004;5:117-126. Available from: https://dergipark.org.tr/tr/pub/trakyafbd/issue/23037/246276
- Shivanna KR. Pollen biology and biotechnology. CRC Press; 2019. Available from: https://doi.org/10.1201/9780429187704
- Stosser R, Hartman W, Anvari S. General aspects of pollination and fertilization of pome and stone fruits. Acta Hort. 1996;423:15-21. Available from: https://doi.org/10.17660/ActaHortic.1996.423.1
- Chkhartishvili N, Vashakidze L, Gurasashvili V, Maghradze D. Type of pollination and indices of fruit set of some Georgian grapevine varieties. Vitis. 2006;45:153. Available from: https://www.researchgate.net/publication/233382555_Type_of_pollination_and_indices_of_fruit_set_of_some_Georgian_grapevine_varieties
- Sharafi Y. Pollen germination, tube growth and longevity in some cultivars of Vitis vinifera L. Afr J Microbiol Res. 2011;5(9):1102-1107. Available from: http://dx.doi.org/10.5897/AJMR11.168
- Vouillamoz JF, McGovern PE, Ergul A, Söylemezoğlu G, Tevzadze G, Meredith CP, Grando MS. Genetic characterization and relationships of traditional grape cultivars from Transcaucasia and Anatolia. Plant Genet Resour. 2006;4:144-158. Available from: http://dx.doi.org/10.1079/PGR2006114
- Baby T, Gilliham M, Tyerman S, Collins C. Differential fruitset between grapevine cultivars is related to differences in pollen viability and amine concentration in flowers. Aust J Grape Wine Res. 2016;22:149-158. Available from: http://dx.doi.org/10.1111/ajgw.12191
- Padureanu S, Patras A. Palynological characterization, germination potential and pollen tube growth of direct producer hybrids Noah and Othello (Vitis genus). Flora. 2018;240:58-67. Available from: http://dx.doi.org/10.1016/j.flora.2018.01.005
- García-Breijo F, Armiñana JR, Garmendia A, Cebrián N, Beltrán R, Merle H. In vivo pollen tube growth and evidence of self-pollination and prefloral anthesis in cv. Macabeo (Vitis vinifera L.). Agriculture. 2020;10:647. Available from: https://doi.org/10.3390/agriculture10120647
- Song J, Mavraganis I, Shen W, Yang H, Patterson N, Wang L, et al. Pistil-derived lipids influence pollen tube growth and male fertility in Arabidopsis thaliana. Plant Physiol. 2024;kiae276. Available from: https://doi.org/10.1093/plphys/kiae276
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
