International Journal of Sexual and Reproductive Health Care
Modifying Factors of the Incidence of Type 1 Diabetes Mellitus in Children under 18 Years of Age in Northern Spain in the Last 3 Decades
Sandra Maeso Méndez1,2, Rut Gago Martín3, Eider Jauregui Benito1, Laura Costa Serra1, Andrea Gainzarain Serna1, Annabel Prigent Diaz1, Carlos Sola Sarabia4, Mikel Ogueta Lana5 and Ignacio Díez López2,6,7*
2Section of Childhood Endocrinology, Pediatrics Service, Áraba University Hospital, OSI Araba Vitoria-Gasteiz, Álava, Spain
3CS Zabalgana OSI Araba Vitoria-Gasteiz, Álava, Spain
4Department of Health, Basque Government, Spain
5Department of Health and Quality Information, Basque Government of Spain
6Department of Pediatrics, UPV-EHU, Vitoria-Gasteiz, Álava, Spain
7BIOARABA. Investigation Institute, Child and Youth Growth and Metabolism Group, Vitoria-Gasteiz, Álava, Spain
Cite this as
Méndez SM, Martín RG, Benito EJ, Serra LC, Serna AG, Diaz AP, et al. Modifying Factors of the Incidence of Type 1 Diabetes Mellitus in Children under 18 Years of Age in Northern Spain in the Last 3 Decades. Int J Sex Reprod Health Care. 2024;7(1):033-039. Available from: 10.17352/ijsrhc.000047Copyright Licence
© 2024 Méndez SM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: Type 1 Diabetes Mellitus is a chronic disease of autoimmune etiology that is highly prevalent in childhood. In the last decade, the possibility of the influence of the Bacillus Calmette-Guérin vaccine, different autoimmune diseases, modification of the immune response, and COVID19 itself of a simple population change due to immigration.
Objectives: The objective of this work is to study the incidence of Type 1 Diabetes Mellitus in a cohort of children from Euskadi, north of Spain in the last 15 years.
Methods: For the epidemiological study in Euskadi, a comparison of accumulated incidences between vaccinated and unvaccinated population groups was made.
Results: The results of our study were not conclusive, but we believe that it is necessary to continue with long-term studies to eliminate bias.
Introduction
Diabetes Mellitus type 1 (DM-1) is a very common chronic disease in childhood. It is an autoimmune disease in which T lymphocytes destroy the beta cells of the pancreas, generating a decrease in insulin levels. Its incidence in Spain is around 10-12 cases per 100,000 children under 14 years of age, with two characteristic age peaks: between 4 and 6 years and between 10 and 14 years [1]. In the specific case of the Basque Country, its incidence in children under 14 years of age ranges between 9.5 and 16 cases per 100,000 children, being minimum in the age group of 0-5 years and maximum between 13 years - 14 years. Furthermore, in the pediatric population, no differences in incidence are found in terms of sex [2].
In the last decade, the possibility that the Bacillus Calmette-Guérin (BCG) vaccine could be an effective alternative for the treatment of DM-1 through its immunomodulatory action has been studied. In addition, it is capable of generating changes in glucose metabolism to ultimately reduce blood glucose levels.
BCG was eliminated from the vaccination schedule of the Basque Country on January 1, 2013, being the only Spanish Autonomous Community that used it for all newborns with a single dose at one month of age. We believe, therefore, that we have a good scenario to assess whether or not its use influences the incidence of DM-1.
On the other hand, we suffered the well-known COVID-19 pandemic at the beginning of 2020. There are multiple studies that have postulated a relationship between this infection and multiple immunological changes (from reactive thromboses, SIMPED syndrome, etc.)
Furthermore, we cannot forget that in the last 15 years (EUSTAT data web https://www.eustat.eus/indice.html) our region, like the rest of the country, has seen a significant increase in the population minor of immigrant origin. This sociocultural change can also lead to other forms of illness and differences in the prevalence of pathologies that influence the ethnic origin of people [2].
These authors have published previous comparison data between fully exposed cohorts and a partially unexposed cohort in Mendez, et al. [3] in the year 2024. Part of the data used in this original comes from this work.
Material and methods
We studied the number of new cases of DM-1 in the Basque Country in children under 19 years of age in the period between the years 2012 and 2022 (that is, an entire decade after the withdrawal of the BCG). The study data was provided by the Department of Public Health and the Department of Health and Quality Information of the Basque Public Health Service (named Osakidetza).
Criteria inclusion: new cases debut of DM type 1 and age below 19 years.
Excluded: others cases of diabetes or ages
The data included, for each year, the total number of new cases by age range (0 to 19 years) and by sex (male, female). As well as the distribution by health areas. Likewise, through the website of the Basque Institute of Statistics (named EUSTAT) (free access from https://www.eustat.eus/indice.html) epidemiological information was extracted on the quantification of the population under 19 years of age in the Basque Country for each of the years and included in the study.
Using the study data, a descriptive analysis of the sample was carried out and cumulative incidence calculations (number of cases/population data from EUSTAT) were made for each of the years.
Likewise, EUSTAT and OSAKIDETZA were used to investigate the incidence of other diseases in the region, immigration rate, and COVID-19 data [2].
Finally, for data comparison, the mean was calculated and Student’s t-test was carried out as a statistical test with an inference of p-value 0.05. The SPSS v19.0 statistical tool was used by our statistic team from BIOARABA (the local agency of our hospital).
Results
Results of the descriptive analysis of the study sample
Table 1 shows the population data of people residing < 19 years of age in the Basque Country obtained from the Statistics Institute of the Basque Government from the withdrawal of the vaccine in 2012 until a later decade in 2022.
The average population of the cohort of those under 19 years of age throughout the study decade is almost 400,000 individuals for an average population of 2.5 million inhabitants (16% of the population).
The total number of DM-1 cases that debuted throughout the region in the last decade was 663 cases. No There are significant differences (p:0.18) between groups of different sexes in our population regarding DM-1 debut cases.
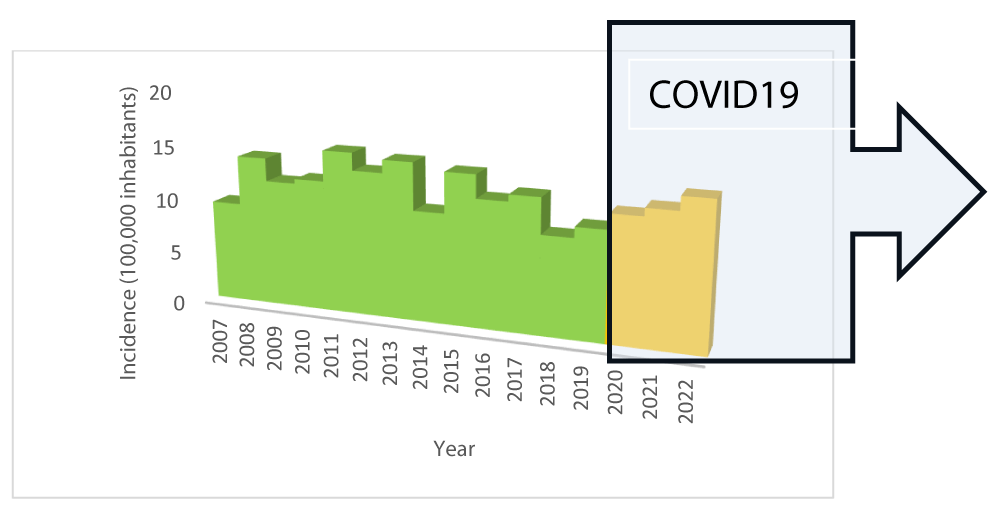
Figure 1 is a representation of the one published by the authors previously [3] showing the start time of the COVID19 pandemic and the relationship with the cumulative incidence of DM1.
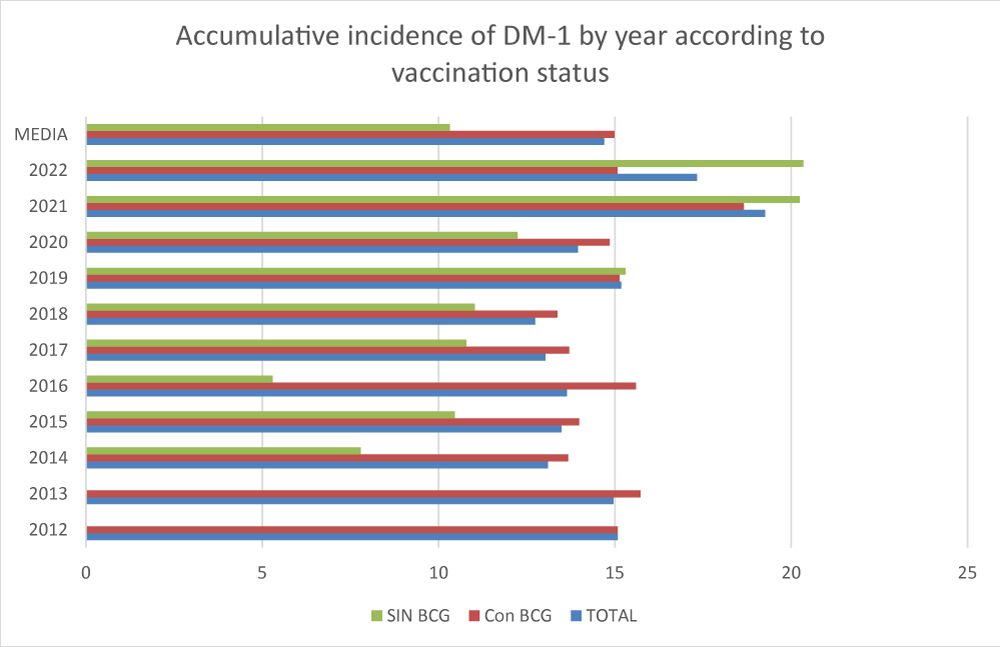
Figure 2 shows the cumulative incidence values of DM-1 in the ten years after the vaccine was withdrawn (2013-2022) according to whether or not the pediatric population had received the vaccine. Let us remember that the BCG vaccine was administered compulsorily to all newborns as a single dose at one month of age and, therefore, after its withdrawal, we will find part of the infant population vaccinated and part unvaccinated.
The average cumulative incidence of DM-1 in the pediatric population under 19 years of age in the six years prior to the withdrawal of the vaccine was 13,607 cases per 100,000 children. In the period 2013-2022, after the withdrawal of the vaccine from the calendar, the average cumulative incidence of DM-1 in the vaccinated child population was 14.99 cases per 100,000 children compared to an average cumulative incidence of 10.32 cases per 100,000 unvaccinated children. In this case, no statistically significant differences were found (p:0.08) although there is a tendency to increase cases among the unvaccinated compared to the vaccinated, especially from the cohort of the year after COVID-19.
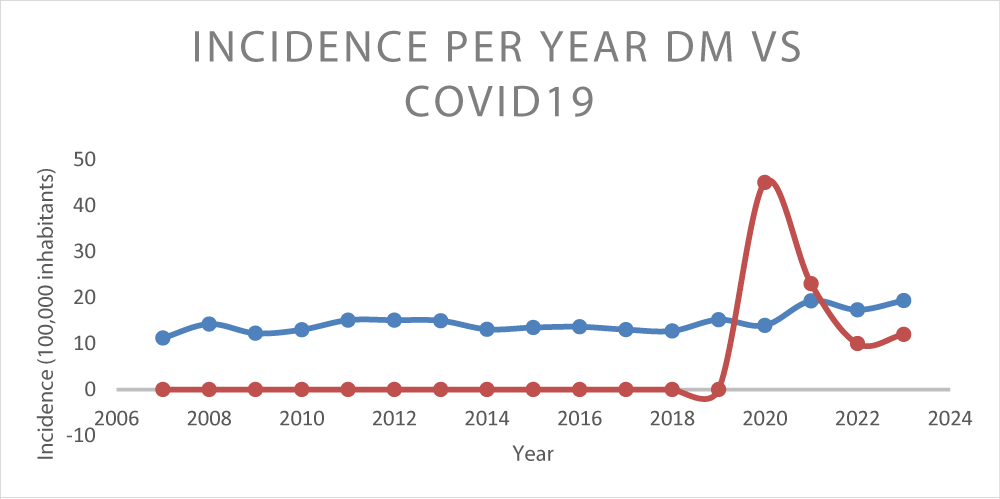
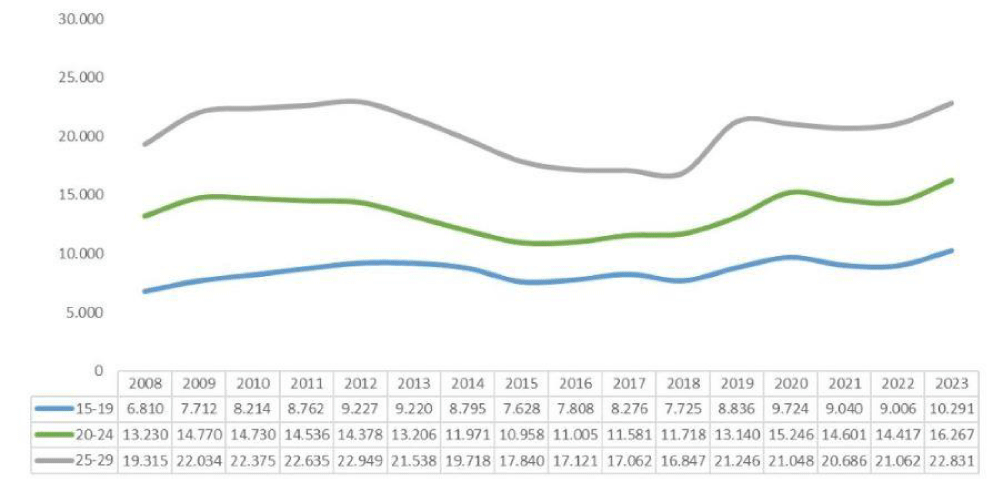
Figure 3 shows a linear trend graph to see the evolution of the accumulated incidence by year associated with COVID-19 cases.
The incidence of cases each year appears quite stable but with an upward trend since 2018. There is a peak in cases of type 1 DM after the COVID19 pandemic. The subsequent upward trend is significant.
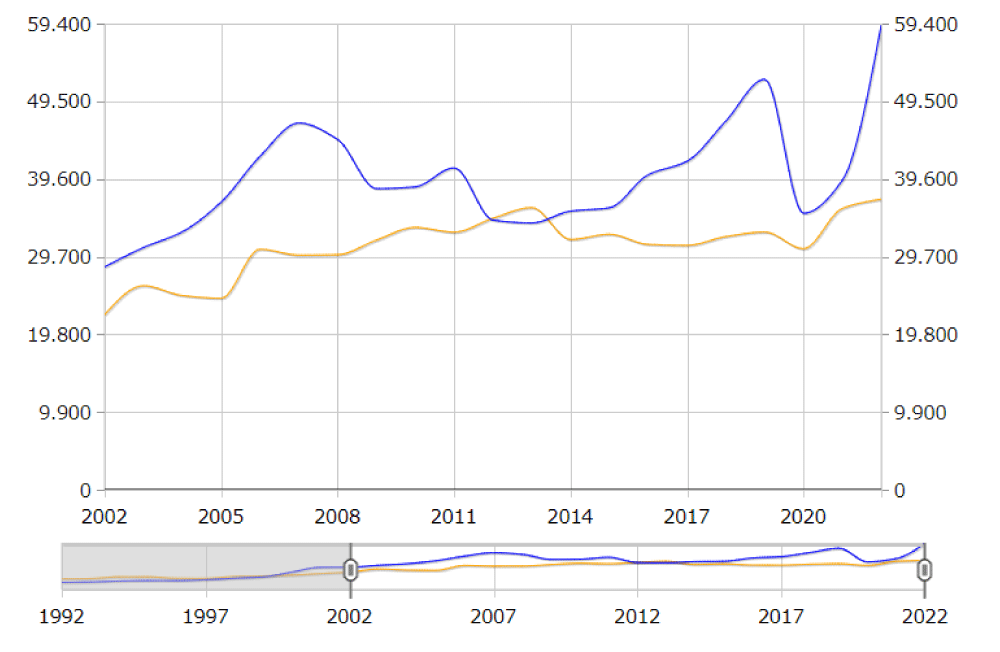
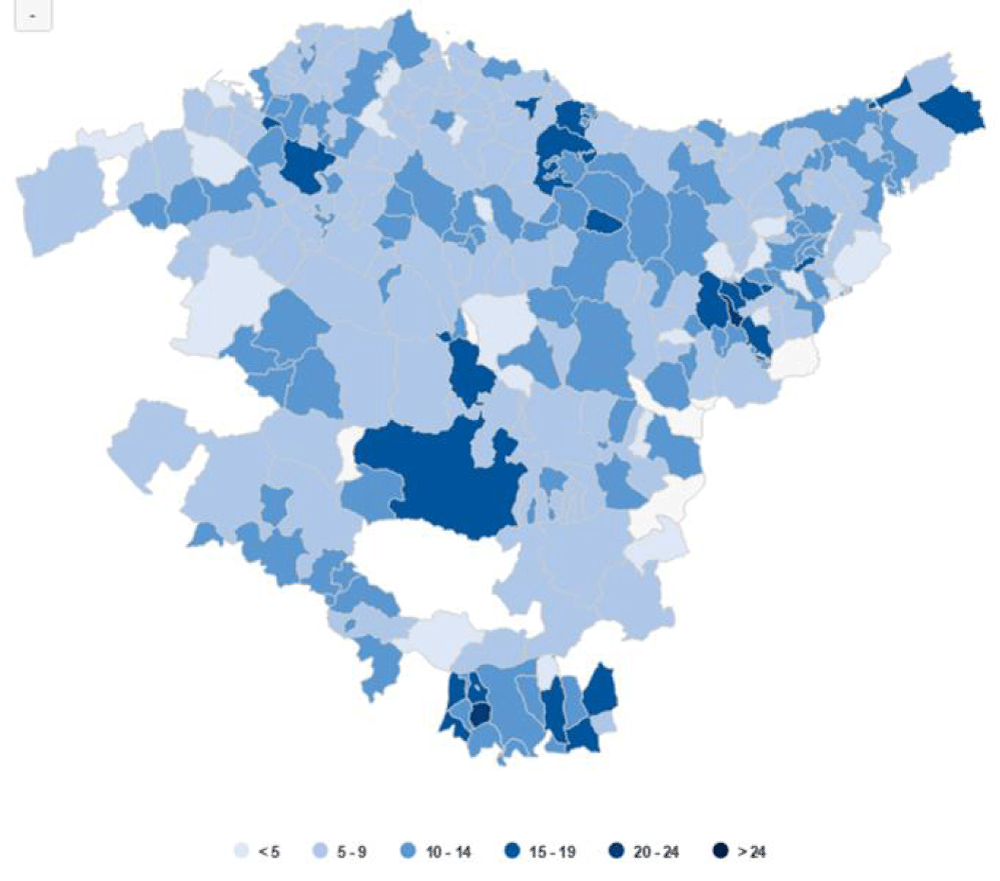
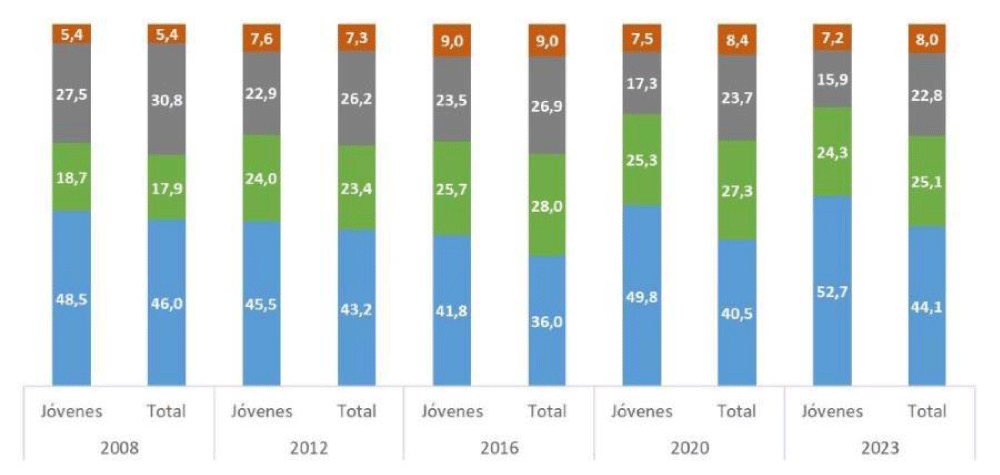
Regarding the proportion of minors of foreign origin in Euskadi, these have also increased in recent years, reaching an average of 16% in recent years (Figure 4). The distribution is asymmetric according to territories, with a more specific weight in the capitals and some rural areas (Figure 5).
The increase in the number of minors of foreign origin or whose parents are foreign has shown a constant increase in recent years (Figure 6).
Most of the foreign community come from territories where the prevalence of infectious diseases such as TB is higher (native TB territories), such as areas of the Middle East, Maghreb, or Latin American countries (Figure 7). In addition to the epidemiological factors of these countries of origin, there are also genetic factors that can explain a higher rate of DM-1 in these groups (1,2).
Discussion
The BCG vaccine was first synthesized in 1921 from a strain of Mycobacterium bovis. It is a live attenuated vaccine that is used, primarily, to protect against tuberculosis [4].
Traditionally, it has been considered that the mechanism by which vaccines are capable of generating a lasting immune response is through adaptive immunity. However, there is increasing evidence of the existence of immunological memory in innate immunity. In this sense, it is postulated that every time innate immune cells are exposed to an antigen, they are “trained” for subsequent exposures to said antigen. Therefore, this type of immunological memory is called trained immunity [5,6].
It has been observed that thanks to trained immunity, the BCG vaccine could prevent infections caused by pathogens not related to tuberculosis that are responsible for neonatal sepsis or lower airway infections in childhood. Likewise, there are currently two studies in development that seek protection against infection by the new SARS-CoV2 coronavirus with the BCG vaccine. But its use is also being investigated for the treatment or prevention of other infectious processes, but also autoimmune, allergic, or oncological processes, although with very different results [7,8].
In tuberculosis infection, the innate immune response plays a very important role as it is the first line of defense against infection [9-11]. Thus, macrophages have a functional duality: on the one hand, they are responsible for phagocytosing mycobacteria; and, on the other hand, they present the protein antigens resulting from phagocytosis to the T lymphocytes. These lymphocytes are responsible for producing different substances, such as TNF-alpha, which activate the granulomatous inflammatory response to prevent the progression of the infection [12]. Based on the pathophysiological mechanism of tuberculosis infection, we can assume that the BCG vaccine is also capable of stimulating the production of TNF without being toxic to the body. This fact opens the door to the possibility of using BCG against the treatment of DM-1.
A study carried out by Faustman, et al. (2012) demonstrates that the BCG vaccine administered repeatedly in small doses selectively decreases autoimmune T lymphocyte populations and, in addition, they observe an increase in C-peptide levels. [13-15].
Infection with Mycobacterium tuberculosis has been observed to be more frequent in some age populations, especially in some countries with a higher incidence [16], where either there is no vaccination or exposure to BCG is early [17]. These countries (Maghreb, Africa, Asia) are the main origin of people emigrating to Europe in general and Spain in particular. Therefore, the prevalence of BCG carriers and/or patients is much higher in these groups than in the native population [18].
The incidence of TB in the immigrant population is so high that the Basque Country has modified its non-vaccination pattern in favor of vaccination and active detection of the disease in immigrant people [19].
After the COVID19 pandemic, several authors have shown the possible relationship with an increase not only in autoimmune diseases in general but in DM-1 in particular [20]. It has been postulated an increase in autoimmune activity after COVID19 infection [21], a delay in diagnosis, an increase in the prevalence of ketoacidosis [22,23], and other causes yet to be determined.
On the other hand, the relationship between geographical origin and greater or lesser prevalence of diseases, including DM-1 [24], is known, as well as the socioeconomic conditions and origin of population groups, including pediatrics [25], have an influence on the prevalence of different diseases. Euskadi is not immune to all this and in the last 15 years, the subject of our study, the number of people and children from diverse origins has increased (EUSTAT). This may contribute to a bias when interpreting population and epidemiological studies.
The incidence of DM-1 in the Basque Country remains low compared to published population and incidence studies [1,2], but in our population, there is an upward trend, which is not statistically significant at the moment. This increase in cases could be due to various factors to be considered:
- A real increase in the incidence of cases in the non-BCG exposed cohort vs. the exposed one. As the unexposed population within the cohorts has more population weight, the incidence of cases could increase.
- The effect of a positive bias after the COVID-19 pandemic [7,8], since this infection could have different effects on the development and evolution of autoimmune diseases, including DM-1 itself.
- Population changes experienced in our region in the last 10 years. The immigration of people from other latitudes is a growing fact in Europe and more significantly in various regions of Spain. It is known [2,3] that the prevalence of diseases with an autoimmune nature, including DM-1, can be influenced by population changes, associated with the population increase of ethnic groups with a higher prevalence (due to genetic and phenotypic bases) of autoimmune diseases. , as is the case of the Maghreb and Latin American populations.
‘Future directions’ of this research could be:
It is necessary to add more years to our study.
Perhaps studying this situation in other regions of Spain.
To study what happens in other ages as well.
The limitations of our work include other variables that we did not have information on, such as antibodies, weight, time from symptoms to debut, severity of debut (pH), and others. Also, the information on the people who have a debut out of our region but are from our region (minor report), and others.
Conclusion
The main conclusion of this study is that the BCG vaccine could have benefits in the control of DM-1 thanks to the selective elimination of the autoimmune T lymphocytes responsible for the disease and, also, the activation of regulatory T lymphocytes through epigenetic changes. Likewise, it promotes changes in carbohydrate metabolism that help control blood glucose levels.
Similarly, assessing the heterologous effect of BCG, its use in other autoimmune diseases, and even in allergic, infectious, and oncological processes could be considered.
However, there are still many questions to be clarified. The vast majority of studies developed so far do not establish the number of times it is necessary to vaccinate patients or with what dose it should be done. It is also not clear how long it takes for the systemic effect of BCG to be observed in DM-1.
Our study has several limitations. First, we did not apply any sample size calculation and our study sample may not be representative. Furthermore, this is a study focused on a single Autonomous Community and, therefore, the results may not be extrapolated to other geographical areas. In this sense, as we mentioned previously, DM-1 in our community has a maximum incidence between 13 years - 14 years of age and a minimum of 0 - 5 years. In the second part of the study, the children who had not received the vaccine were younger than those who had received it and this could represent a bias when interpreting the data.
Nor did we control, through the data obtained, the ethnic origin of the people affected by DM-1, which could influence the genotypic expression of DM-1 or even having suffered or been exposed to the native BCG itself.
For all these reasons, we consider that it is necessary to continue more studies with a larger number of participants, of different ages, in different geographical areas, and with a variable duration of the disease. It is important to know exactly whether BCG could be an effective, safe, and long-lasting treatment for a chronic disease as prevalent in childhood as DM-1 [26].
- Conde Barreiro S, Rodríguez Rigual M, Bueno Lozano G, López Seguiro JP, González Pelegrín B, Rodrigo Val MP, et al. Epidemiology of type 1 diabetes mellitus in children in Spain. An Pediatr (Barc). 2014;81(3):189.e1-189.e12. Available from: https://doi.org/10.1016/j.anpedi.2013.12.010
- Working group of the Clinical Practice Guide on Diabetes Mellitus Type 1. Clinical Practice Guide on Diabetes Mellitus Type 1. Quality Plan for the National Health System of the Ministry of Health and Social Policy. Health Technology Assessment Agency of the Basque Country-Osteba; 2012. Clinical Practice Guidelines in the SNS: OSTEBA no. 2009/10. Available from: https://www.scirp.org/reference/referencespapers?referenceid=3339581
- Méndez SM, Martin RG, Benito EJ, Serra LC, Serna AG, Diaz AP, et al. Influence of the Bacillus Calmette-Guerin Vaccine on the Incidence of Type 1 Diabetes Mellitus Onset in a Child and Adolescent Population. J Diabet Clin Endocrinol. 2024;105. Available from: http://dx.doi.org/10.20944/preprints202405.0906.v1
- Kühtreiber WM, Tran L, Kim T, Dybala M, Nguyen B, Plager S, et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations. Npj Vaccines. 2018;3(23):1-14. Available from: https://www.nature.com/articles/s41541-018-0062-8
- Shann F. The nonspecific effects of vaccines and the expanded program on immunization. J Infect Dis. 2011;204:182-184. Available from: https://doi.org/10.1093/infdis/jir244
- Goodridge HS, Ahmed S, Curtis N, Kollmann TB, Levy O, Netea MG, et al. Harnessing the beneficial heterologous effects of vaccination. Nat Rev Immunol. 2016;16:392-400. Available from: https://doi.org/10.1038/nri.2016.43
- Ristori G, Romano S, Cannoni S, Visconti A, Tinelli E, Mendozzi L, et al. Effects of Bacille Calmette-Guerin after the first demyelinating event in the CNS. Neurology. 2014;82:41-48. Available from: https://doi.org/10.1212%2F01.wnl.0000438216.93319.ab
- Arnoldussen DL, Linehan M, Sheikh A. BCG vaccination and allergy: a systematic review and meta-analysis. J Allergy Clin Immunol. 2011;127:246-253. Available from: https://doi.org/10.1016/j.jaci.2010.07.039
- Diego Ricardo Muñoz Cendales DG, Cuca Suárez LE. Cytotoxic compounds of plant origin and their relationship with inhibitory proteins of apoptosis (IAP). Rev Colomb Cancerol. 2016;20(3):124-134. Available from: https://www.revistacancercol.org/index.php/cancer/issue/view/14?time=1721032725?time=1723193259
- Ryu S, Kodama S, Ryu K, Schoenfeld DA, Faustman DL. Reversal of established autoimmune diabetes by restoration of endogenous beta cell function. J Clin Invest. 2001;108:63-72. Available from: https://doi.org/10.1172/jci12335
- Ban L, Zhang J, Wang L, Kuhtreiber W, Burger D, Faustman DL. Selective death of autoreactive T cells in human diabetes by TNF or TNF receptor 2 agonism. Proc Natl Acad Sci USA. 2008;105:13644-13649. Available from: https://doi.org/10.1073%2Fpnas.0803429105
- García MA, Sarmiento ME, Acosta A. Anti-tuberculosis immunity and its application in the development of vaccine candidates. VacciMonitor. 2009;18(1):25-34.
- Faustman DL, Wang L, Okubo Y, Burger D, Ban L. Proof-of-Concept, Randomized, Controlled Clinical Trial of Bacillus-Calmette-Guerin for Treatment of Long-Term Type 1 Diabetes. PLoS ONE. 2012;7(8). Available from: https://doi.org/10.1371/journal.pone.0041756
- Wang L, Lovejoy NF, Faustman DL. Persistence of prolonged C-peptide production in type 1 diabetes as measured with an ultrasensitive C-peptide assay. Diabetes Care. 2012;35:465-470. Available from: https://doi.org/10.2337/dc11-1236
- Okubo Y, Torrey H, Butterworth J, Zheng H, Faustman DL. Treg activation defect in type 1 diabetes: correction with TNFR2 agonism. Clin Transl Immunol. 2016;5. Available from: https://doi.org/10.1038/cti.2015.43
- Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, et al. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med. 2007;204:2159–2169. Available from: https://doi.org/10.1084%2Fjem.20062105
- Yadav V, Dwivedi VD, Bhattacharya D, Mittal A, Moodley P, Daset G. Understanding the host epigenetics in Mycobacterium tuberculosis infection. J Genet Genome Res. 2015;2:016. Available from: http://dx.doi.org/10.23937/2378-3648/1410016
- Vasiliu A, Köhler N, Altpeter E, Ægisdóttir TR, Amerali M, de Oñate WA, et al. Tuberculosis incidence in foreign-born people residing in European countries in 2020. TBnet. Euro Surveill. 2023;28(42):2300051. Available from: https://doi.org/10.2807/1560-7917.es.2023.28.42.2300051
- Vasco EJ-G. Manual de Vacunaciones, Manual de vacunaciones - Enfermedades, vacunas y vigilancia epidemiológica - Departamento de Salud - Gobierno Vasco - Euskadi.eus. 2024. Available from: https://www.euskadi.eus/informacion/manual-de-vacunaciones/web01-a2gaixo/es/.
- Arts RJ, Carvalho A, La Rocca C, Matarese G, Van Crevel R, Netea MG. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 2016;17:2562-2571. Available from: https://doi.org/10.1016/j.celrep.2016.11.011
- Varol F, Ozyilmaz LGB, Sahin EG, Can YY, Altas U, Cam H. Does the severity of diabetic ketoacidosis in children with type 1 diabetes change during the COVID-19 pandemic? A single-center experience from a pediatric intensive care unit. North Clin Istanbul. 2022 Oct 20;9(5):429-435. Available from: https://doi.org/10.14744/nci.2022.09634
- Loh C, Weihe P, Kuplin N, Placzek K, Weihrauch-Blüher S. Diabetic ketoacidosis in pediatric patients with type 1- and type 2 diabetes during the COVID-19 pandemic. Metabolism. 2021;122:154842. Available from: https://doi.org/10.1016%2Fj.metabol.2021.154842
- Al-Qahtani MH, Bukhamseen FM, Al-Qassab AT, Yousef AA, Awary BH, Albuali WH, Alkhalifa ZM, Yousef HA. The impact of COVID-19 lockdown on the incidence of type 1 DM and the glycemic control of diabetic children: Findings from a teaching hospital, Saudi Arabia. Rev Diabet Stud. 2022;18(3):152-156. Available from: https://doi.org/10.1900%2FRDS.2022.18.152
- Kashfi K, Anbardar N, Asadipooya A, Asadipooya K. Type 1 diabetes and COVID-19: A literature review and possible management. Int J Endocrinol Metab. 2023 Oct 23;21(4). Available from: https://doi.org/10.5812%2Fijem-139768
- Dariya B, Chalikonda G, Srivani G, Alam A, Nagaraju GP. Pathophysiology, etiology, epidemiology of type 1 diabetes and computational approaches for immune targets and therapy. Crit Rev Immunol Actions. 2019;39(4):239-265. Available from: https://doi.org/10.1615/critrevimmunol.2019033126
- Dickson CA, Ergun-Longmire B, Greydanus DE, Eke R, Giedeman B, Nickson NM, et al. Health equity in pediatrics: Current concepts for the care of children in the 21st century. Dis Mon. 2024;70(3):101631. Available from: https://doi.org/10.1016/j.disamonth.2023.101631
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
