International Journal of Sexual and Reproductive Health Care
Factors Associated with the Occurrence of Urogenital Bacterial Infections in Pregnant Women at a Reference Hospital in Cameroon
Ebong SB1-3*, Tchugo TV2, Ekwi DA3, Lendem I3, Mballa A3,4, Nke M3, Edima HC3, Bika CE1, Adiogo D4 and Eboumbou EC4
1Unit of Physiology and Medicine of Physical and Sports Activities, Faculty of Sciences, University of Douala, Cameroon
2Department of Clinical Biology, Institute of Health Applied Sciences, Douala, Cameroon
3Department of Biomedical and Medical Sciences, Faculty of Sciences, University of Ebolowa, Cameroon
4Department of Biological Sciences, Faculty of Medicine and Pharmaceutical Sciences, University of Douala, Cameroon
Cite this as
Ebong SB, Tchugo TV, Ekwi DA, Lendem I, Mballa A, Nke M, et al. Factors Associated with the Occurrence of Urogenital Bacterial Infections in Pregnant Women at a Reference Hospital in Cameroon. Int J Sex Reprod Health Care. 2024;7(1):048-053. Available from: 10.17352/ijsrhc.000049Copyright Licence
© 2024 Ebong SB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: According to the World Health Organization (WHO), the most common bacterial infections in pregnant women are urinary tract infections (46%) and vaginal infections (26%). Pregnant women are at risk of contracting these dangerous urogenital bacterial infections, with more or less serious consequences for the health of both mother and child. The aim of this study was to identify the factors associated with the occurrence of urogenital bacterial infections in pregnant women attending antenatal clinics.
Methodology: A cross-sectional, analytical study was conducted from May 13 to June 14, 2024, at Douala Laquintinie Hospital. All pregnant women attending antenatal clinics regardless of gestational trimester and consenting to the study were included. Data were analyzed using SPSS 20, R 3.4, and Epi info 7.8. Chi-square, Fischer, and logistic regression tests were performed.
Results: A total of 153 pregnant were included in this study. The incidence of urogenital infections in this population was 42%. Infectious agents were E-coli (18.95%), Klebsiella pneumoniae (7.83%), Gardnerella vaginalis (24.44%), and Streptococcus B (2.22%). Self-medication (OR=17.72; p = 0.0003), drying underwear inside the house (OR=6.53; p = 0.00081), use of traditional toilets (OR=2.65; p = 0.00001), surgical history (OR=2.10; p = 0.01), vaccination status (OR=2.11; p = 0.003), use of borehole and well water (OR=0.02; p = 0.002), frequency of sanitary pad changing (OR=1.13; p = 0.011), frequency of sexual intercourse (OR=1.32; p = 0.012), traditional purging (OR=0.06; p = 0.0001), poor personal hygiene (OR=0.2; p = 0.0031), multiple sexual partners (OR=1.8; p = 0.022) and tight underwear (OR=0.4; p = 0.01) were associated with the occurrence of urogenital bacterial infections.
Conclusion: Urogenital bacterial infections during pregnancy are dangerous. Knowledge of the factors associated with their occurrence will enable the implementation of appropriate control strategies against these infections.
Abbreviations
AB: Asymptomatic Bacteriuria; AIDS: Acquired Immunodeficiency Syndrome; ANC: Antenatal Care; ATB: Antibiotic; ATCD: Antecedents; Bg-: Gram-negative Bacillus; Bg+: Gram-positive Bacilli; BMI: Body Mass Index; CBEU: Cytobacteriological Examination of Urine; Cg+: Gram-positive Cocci; CLED: Cystine Lactose Electrolyte Deficient; E-coli: Escherichia Coli; CVS: Cervico-vaginal Sampling; EMB: Eosin Methylene Blue; GV: Gardnerella Vaginalis; HIV: Human Immunodeficiency Virus; OR: Odd ratio; PSD: Premature Spontaneous Delivery; STD: Sexually Transmitted Disease; STI: Sexually Transmitted Infection; TV: Trichomonas Vaginalis; UD: Urine Dipstick; UTI: Urinary Tract Infection; WHO: Word Health Organization
Introduction
Bacterial, viral, fungal, and parasitic infections, whether endemic or epidemic, affect human beings, particularly the pregnant, for whom they represent a real danger. They account for around 15% of pregnancies, i.e. some 100,000 infections per year, and 2% of fetuses, i.e. 15,000 per year [1]. Worldwide, 2% to 10% of women attending antenatal clinics are affected by gravid bacterial infections [2]. According to the WHO, more than 1,000 women worldwide die every day from complications related to pregnancy and/or childbirth, over half of them in sub-Saharan Africa and 1/3 in South Asia [3]. In 2019, the most common bacterial infections among pregnant were urinary tract infections (46%) and vaginal infections (26%). In West Africa, a study carried out on vaginal infections in pregnant women at the Sokodé regional hospital in Togo revealed that pregnant in this town were exposed to vaginal infections with the predominance of G. vaginalis and Candida sp [4]. In Cameroon, a study of pregnant in Yaoundé revealed that 6.70% were carriers of group B beta-hemolytic Streptococcus [5]. Another study carried out in Cameroon on a number of women, including pregnant at Bonassama district hospital in Douala, revealed that 5.56% of these pregnant suffered from G. vaginalis infection [6].
Pregnancy favors severe forms of the disease for the woman and even the child. Bacterial (maternal) infection is a real public health problem, as it can have more or less serious consequences for the health of the mother and the unborn child, depending on the causal agent and the term of the pregnancy. Mother-to-child transmission of these urogenital bacterial infections can lead to fetal malformation or lesions, neonatal infection, and long-term disability after the baby’s birth [7]. Asymptomatic bacteriuria is 20 to 30 times more likely to develop pyelonephritis, 50% more likely to have a low-birth-weight baby, and twice as likely to have a premature baby [8].
Douala, a cosmopolitan, densely populated city with squatter settlements, promiscuity, insalubrity, under-education, unwanted pregnancies, poverty, lack of drinking water, and unemployment, is a major hotbed of infection transmission, including bacterial infections, offering ideal conditions for the proliferation and transmission of these germs. The nuisance of these infectious agents in pregnant women is generally facilitated by numerous socio-environmental, demographic (age, place of residence, profession...), behavioral (number of sexual partners, self-medication, water supply, type of intimate shower, type of latrine used, knowledge, poor hygiene...) and gynecological (parity, surgical/medical or abortion history, vaccination status...) factors. For this reason, we felt it was important to carry out this study to identify the factors associated with the occurrence of urogenital bacterial infections in pregnant attending antenatal clinics (ANC) at Douala Laquintinie Hospital.
Materials and methods
Type of study and target population
A cross-sectional, analytical study was conducted (December 2023 to August 2024) on pregnant women received in ANC and seen in the laboratory for urinalysis and Cervico-vaginal Sampling (CVS) of any gestational trimester, present during the data collection period and consenting to the study were included. We used non-probability, simple random, and consecutive sampling. The sample size was determined by Schwartz’s formula: N = Z2PQ/I2. We obtained prior ethical clearance (No 4339 CEI-UDo /06/24/M) from the institutional ethics committee of the University of Douala, administrative authorization (No 057/ARC/MINSANTE/DHL/SG) from the Director of Douala Laquintinie Hospital and informed consent from the participants. Data were collected using an anonymous questionnaire and coded to preserve the anonymity of the participants and guarantee the confidentiality of the information gathered. They were then recorded on a Microsoft Excel data entry sheet and analyzed using SPSS version 20, Epi info version 7.8, and R version 3.4. Qualitative data were presented as frequencies and percentages. Quantitative data were presented in means and standard deviations. Data were compared using the Chi-square or Fischer’s exact test for qualitative variables, and Student’s t-test for quantitative variables. Correlations between variables were investigated using multivariate analysis tests. Odds ratios were determined to assess risk levels. Any difference was statistically significant for p<0.05.
Laboratory analysis procedures
Preparation of culture media: Several culture media were prepared 3 days before use, kept cold between 2 and 8 °C, then inoculated to allow growth of the various bacteria likely to be present in the samples; CLED (Cystine Lactose Electrolyte Deficient) for cytobacteriological examination of urine (CBEU), EMB (Eosin Methylene Blue) for Cervico-vaginal Sampling (CVS), Chapman with mannitol for staphylococcus testing, GS (Fresh Blood Agar) for streptococcus growth, choc (Cooked Blood Agar) for Neisseria gonorrheae growth. Once the media had been prepared, quality control tests (sterility and fertility) were carried out. Biological material (urine and cervico-vaginal secretion) was collected in compliance with sampling standards and conditions. Macroscopic, microscopic, and biological analyses were carried out.
Results
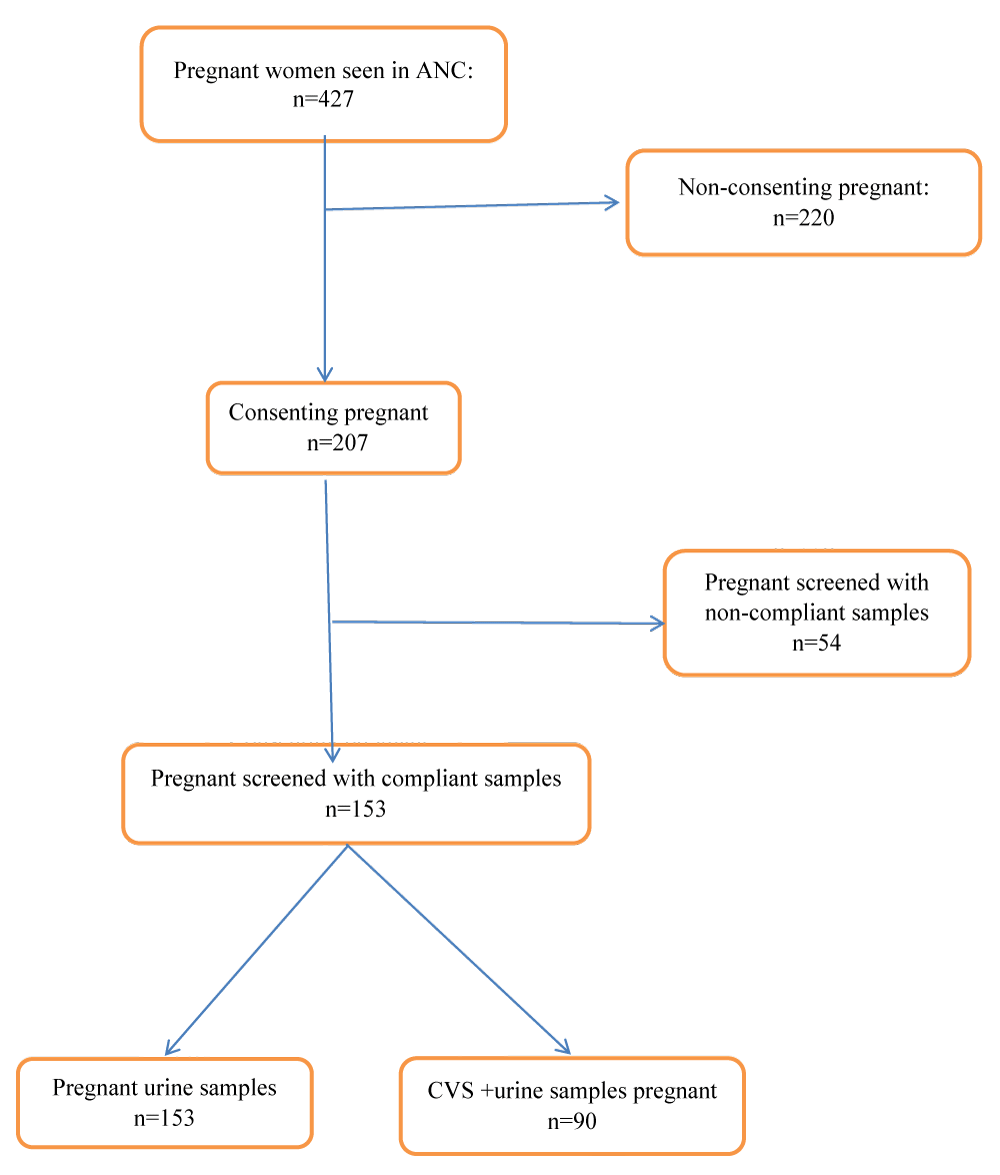
Figure 1. Flow chart of pregnant women attending antenatal clinics at Laquintinie Hospital, Douala.
Sociodemographic characteristics
Of 427 pregnant seen in ANC, 153 patients were included in this study (Figure 1). The mean age of the patients was 30.2 ± 12. The most represented age group was 26-35 (49.67%), with informal sector workers the most numerous. The majority lived in the Douala 3rd arrondissement (45.75%). They were mostly Christian (90.20%) and single (50.33%), with a university education (50.33%) (Table 1).
Incidence of urogenital infections
Of 153 pregnant screened, 65 (42%) tested positive for urogenital infections and 41 (26.80%) for urinary bacterial infections. Of the 90 leucorrhoea samples taken, 24 (26.66%) were positive. Enterobacteriaceae such as E-coli (18.95%) and Klebsiella pneumoniae (7.83%) were the most common microorganisms identified in urine. Microorganisms isolated from leukorrhea included Gardnerella vaginalis (22.22%), Gardnerella vaginalis-Trichomonas vaginalis co-infection (2.22%), and Streptococcus B (2.22%).
According to socio-demographic characteristics, the age group most affected by urogenital infections was 26-35 (19.61%). Most of them worked in the informal sector (16.99%) and lived in the Douala 3rd arrondissement (20.92%). Married (22.22%) and university-educated (32.02%) women were the most affected by urogenital infections, although no association was established between socio-demographic characteristics and the occurrence of urogenital infections (Table 2).
Factors associated with the occurrence of urogenital infections
Factors associated with the occurrence of urogenital infections
Knowledge of urogenital infections (OR=0.46; p = 0.003), self-medication (OR=17.72; p = 0.000), drying underwear inside the house (OR=6.53; p = 0.000), use of traditional toilets (OR=2.65; p = 0.000), surgical history (OR=2.10; p = 0.010), vaccination status (OR=2.11; p = 0.003), use of borehole and well water (OR=0.02; p = 0.002), frequency of sanitary pad changing (OR=1.13; p = 0.011), frequency of sexual intercourse (OR=1.32; p = 0.012), traditional purging (OR=0.06; p = 0.000), poor personal hygiene (OR=0.2; p = 0.003), multiple sexual partners (OR=1.8; p = 0.022) and tight underwear (OR=0.4; p = 0.01) were associated with the occurrence of urogenital bacterial infections (Table 3).
Discussion
The most frequently reported pregnancy-related infections are urogenital tract infections, which most often cause complications in pregnancy [9]. Although sometimes asymptomatic during pregnancy, the fetus is always placed at high risk of prematurity or low birth weight [10-13]. In our study, 153 pregnant at different stages of pregnancy were included, with the most common age range being 26-35 years, very similar to that found in studies published by Diassana, et al. Epok, et al. who found that the age range of infected pregnant was 25-35 years [11,14-17]. This could be explained by the fact that women in this age bracket are for the most part married and also voluntarily seeking children. This age group is also the most sexually active and probably the most exposed to various infections. 44.44% worked in the informal sector. This could be explained by the difficult socio-economic context; in Cameroon, people tend to work in small trades. These women are sometimes obliged to work in the informal sector to support themselves and their families. The most represented gestational term was the 2nd trimester. This could be explained by the late start of prenatal consultations in our country, linked to several factors [18]. Place of residence and level of education are indicators of a pregnant woman’s physiological state. The higher the level of education, the more abnormal the flora. This may seem contradictory, but it could be explained by the fact that the most educated women were the ones who used antiseptics the most, and consequently, the ones who degraded their vaginal microflora the most, predisposing them to infections. Married women had the most abnormal vaginal microflora. This could be explained by the fact that frequent intercourse causes bacteria to migrate to the urinary tract. The incidence of urinary tract bacterial infections was 26.79%, slightly higher than that found in Algeria by Amara and al in 2022 (2.5 to 17.5%) [10]. This difference could be explained not only by the difference in size (90 leucorrhoea samples in our study versus 124 in theirs) but also by the duration of the study (1 month versus 4 months).
The germs isolated in positive urine samples were gram-negative bacilli, represented mainly by Enterobacteriaceae, with E-coli at the top of the list (18.95%), and followed by Klebsiella pneumoniae (7.83%). The same germs were also found by Stenkvist, et al. [11,19]. Among the germs isolated from positive Cervico-vaginal Swabs (CVS), Gardnerella vaginalis was predominant, as noted by Eunjung Jung, et al. [7]. Two strains of Streptococcus B were also isolated (2.22%), as in a study published by Foumane, et al. but at a higher rate (6.70%) [5,8]. This difference can be explained by the sample size, type, and duration of the study. Two co-infection strains, Gardnerella vaginalis and Trichomonas vaginalis, were isolated. This would indicate the absence of precautions in relation to the type of water used (well water and borehole), place of residence (Douala 3rd and 5th), type of latrine used (traditional and public), and number of sexual partners.
In multivariate analysis, several factors were associated with the occurrence of urogenital infections. Knowledge of urogenital infections (OR=0.46; p = 0.003), self-medication (OR=17.72; p = 0.000), drying underwear inside the home (OR=6.53; p = 0.000), use of traditional toilets (OR=2.65; p = 0.000), surgical history (OR=2.10; p = 0.010), vaccination status (OR=2.11; p = 0.003), use of borehole and well water (OR=0.02; p = 0.002), frequency of sanitary pad changing (OR=1.13; p = 0.011), frequency of sexual intercourse (OR=1.32; p = 0.012), traditional purging (OR=0.06; p = 0.000), poor personal hygiene (OR=0.2; p = 0.003), multiple sexual partners (OR=1.8; p = 0.022) and tight underwear (OR=0.4; p = 0.01) were associated with the occurrence of urogenital bacterial infections. Taking medication without a medical prescription can lead to therapeutic failure due to incorrect dosage. Unsuitable antibiotics can lead to acquired bacterial resistance [20]. This is also the finding of a study by Saga, et al. [17,18]. Drying underwear inside the home increases humidity, creating an environment conducive to the growth of itchy molds and lesions that can be the gateway to bacteria from the digestive tract, such as E-coli. The maintenance of traditional toilets remains a major problem in Africa. Some authors have established a high level of infection transmission through this channel [12,21]. Excessive douching disrupts the functioning of vaginal microflora, as also revealed in a study by Gilstrap, et al. [11]. This would facilitate the development of infections. Other authors have reported contamination via vaginal douches [12,21]. Vaccination status would be a marker for follow-up prenatal consultations [22]. All pregnant whose vaccination status was complete and up to date were the least infected. Some of our participants had developed illnesses such as psoriasis during pregnancy, which would have weakened their immune systems, making them vulnerable to infection. Similarly, those who had already undergone a caesarean section were also infected. Transmission via wet wipes was observed in a study by Pétrin, et al. and Peterson, et al. [12,21]. One of the limitations of this study was not only the sample size but also the type and duration of the study. For this reason, we plan to extend the study to a larger population in the future.
Conclusion
Bacterial urogenital infections during pregnancy are commonplace and remain a major concern because of the potential severity of maternal and fetal harm. A high incidence of urogenital infections has been established. Infectious agents were E-coli, Klebsiella pneumoniae, Gardnerella vaginalis, and Streptococcus B. Self-medication, drying underwear inside the home, use of traditional toilets, surgical history, vaccination status, use of borehole and well water, frequency of sanitary pad change, frequency of sexual intercourse, traditional purging, poor personal hygiene, multiple sexual partners and tight underwear were associated with the occurrence of urogenital bacterial infections.
Availability of data and materials
All data underlying the findings have been presented within the manuscript.
Consent for publication
Consent to publish has been obtained from all included persons in the study.
We are very grateful to the questionnaire respondents who agreed to participate in this study. We express our gratitude to the DLH personnel for their support and cooperation during the survey.
- Tazi A, Poyart C. Bacteriological risk during pregnancy. Rev Francophone Lab. 2007;389:46-48.
- Jonathan S, Muanda TF. Maternal infection can cause spontaneous abortion: response to "Maternal infection can cause spontaneous abortion". CMAJ. 2017;189(31). Available from: https://doi.org/10.1503/cmaj.733203
- World Health Organization. Maternal mortality. 2024; Reminder n°348. Available from: https://www.who.int/news-room/fact-sheets/detail/maternal-mortality
- Tchelougou D, Karou DS, Kpotsra A, Balaka A, Assih M, Bamoke M, et al. Vaginal infections in pregnant women at the regional hospital of Sokodé (Togo). Trop Med Health. 2013;23(1):49-54. Available from: https://doi.org/10.1684/mst.2013.0142
- Foumane P, Mboudou E, Dohbit JS, Nkemayim DC, Tchokoteu PF, Doh AS. Group B Streptococcus beta hemolyticus and maternal-fetal consequences observed at Yaoundé General Hospital. Clin Mother Child Health. 2009;6(1).
- Ngaba GP, Essomba EN, Kedy Koum C, Ndzengue L, Bika C, Adiogo D. Profile of germs involved in cervicovaginal infections in women of childbearing age at Bonassama District Hospital. J Med Pharm. 2014;4(1):400-408.
- Jung E, Romero R, Yeo L, Diaz-Primera R, Marin-Concha J, Para R, et al. The fetal inflammatory response syndrome: the origin of a concept, pathophysiology, diagnosis, and obstetrical implications. Semin Fetal Neonatal Med. 2020;25(4):101-146. Available from: https://doi.org/10.1016/j.siny.2020.101146
- Amara R, Zaidi C, Guerri D, Khennouchi NC. Urinary tract infections in women during pregnancy and postpartum. Université of Larbi Ben M'hidi-Oum El Bouaghi. 2022.
- Pfau A, Sacks T. Effective prophylaxis for recurrent urinary tract infections during pregnancy. Clin Infect Dis. 1992;14:810-814. Available from: https://doi.org/10.1093/clinids/14.4.810
- Edmonds DK. Benign diseases of vagina, cervix and ovary. In: Dewhurst's Textbook of Obstetrics and Gynaecology. 2012:706-714. Available from: https://doi.org/10.1002/9781119979449.ch53
- Gilstrap LC, Leveno KJ, Cunningham FG. Renal infection and pregnancy outcome. Am J Obstet Gynecol. 1981;141:709-716. Available from: https://doi.org/10.1016/s0002-9378(15)33316-0
- Bertrand X, Thouverez M, Bruand L, Bonnin M, Cellier-Julienne G, Chantelat P, et al. Escherichia coli: sensitivity to beta-lactams and genomic diversity of strains isolated in Franche-Comté. Med Infect Dis. 2002;32(1):8-18. Available from: https://doi.org/10.1016/S0399-077X(01)00313-4
- Sweet RL, Gibbs RS. Infectious diseases of the female genital tract. Philadelphia: Lippincott Williams & Wilkins; 2009.
- Diassana H, Kone. Urinary tract infection and pregnancy at the Renée Cisse Maternity Hospital in Hamdallaye. Bamako, Mali: University of Bamako; 2002.
- Epok C. Urinary tract infections in Bamako: epidemiological and etiological aspects. Bamako, Mali: University of Bamako; 1999.
- Saga T, Yamaguchi. History of antimicrobial agents and resistant bacteria. JMAJ. 2009;52(2):103-108. Available from: https://www.med.or.jp/english/activities/pdf/2009_02/103_108.pdf
- Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birthweight. Obstet Gynecol. 1989;73(4):576-582. Available from: https://pubmed.ncbi.nlm.nih.gov/2927852/
- Newton ER, Walters MD, Karram MM. The urinary tract in pregnancy. In: Walters MD, Karram MM, editors. Clinical Urogynecology. St. Louis: Mosby; 1993;388-408.
- Stenkvist K, Dahlen-Nilsson I, Lidin-Janson G, Lincoln K, Oden A, Rignell S, et al. Bacteriuria in pregnancy: frequency and risk of acquisition. Am J Epidemiol. 1989;129(2):372-379. Available from: https://doi.org/10.1093/oxfordjournals.aje.a115140
- Aminov RI. A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol. 2010 Dec 8;1:134. Available from: https://doi.org/10.3389/fmicb.2010.00134
- Jeanmougin P, Le Bel J. Antibiotic therapy in pregnant and lactating women. EMC-Treatise on Medicine. 2014;9(1):1-7.
- Dinh A, Baumann R, Daoul S, Salomon J, Bruyère F, Louis-Bernard. Rules for prescribing urological antibiotics to pregnant women. Adv Urol - FMC. 2009;19(4).
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
